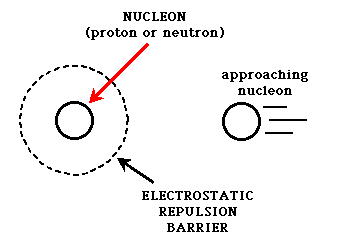
If you consider that the nucleus of all atoms except hydrogen contain more than one proton, and each proton carries a positive charge, then why would the nuclei of these atoms stay together? The protons must feel a repulsive force from the other neighboring protons. This is where the strong nuclear force comes in. The strong nuclear force is created between nucleons by the exchange of particles called mesons. This exchange can be likened to constantly hitting a ping-pong ball or a tennis ball back and forth between two people. As long as this meson exchange can happen, the strong force is able to hold the participating nucleons together. 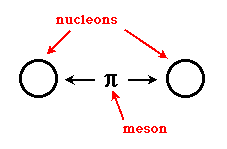 The nucleons must be extremely close together in order for this exchange to happen. The distance required is about the diameter of a proton or a neutron. If a proton or neutron can get closer than this distance to another nucleon, the exchange of mesons can occur, and the particles will stick to each other. If they can't get that close, the strong force is too weak to make them stick together, and other competing forces (usually the electromagnetic force) can influence the particles to move apart. This is represented in the following graphic. The dotted line surrounding the nucleon being approached represents any electrostatic repulsion that might be present due to the charges of the nucleons/particles that are involved. A particle must be able to cross this barrier in order for the strong force to "glue" the particles together.
The nucleons must be extremely close together in order for this exchange to happen. The distance required is about the diameter of a proton or a neutron. If a proton or neutron can get closer than this distance to another nucleon, the exchange of mesons can occur, and the particles will stick to each other. If they can't get that close, the strong force is too weak to make them stick together, and other competing forces (usually the electromagnetic force) can influence the particles to move apart. This is represented in the following graphic. The dotted line surrounding the nucleon being approached represents any electrostatic repulsion that might be present due to the charges of the nucleons/particles that are involved. A particle must be able to cross this barrier in order for the strong force to "glue" the particles together.
In the case of approaching protons/nuclei, the closer they get, the more they feel the repulsion from the other proton/nucleus (the electromagnetic force). As a result, in order to get two protons/nuclei close enough to begin exchanging mesons, they must be moving extremely fast (which means the temperature must be really high), and/or they must be under immense pressure so that they are forced to get close enough to allow the exchange of meson to create the strong force. Now, back to the nucleus. One thing that helps reduce the repulsion between protons within a nucleus is the presence of any neutrons. Since they have no charge they don't add to the repulsion already present, and they help separate the protons from each other so they don't feel as strong a repulsive force from any other nearby protons. Also, the neutrons are a source of more strong force for the nucleus since they participate in the meson exchange. These factors, coupled with the tight packing of protons in the nucleus so that they can exchange mesons creates enough strong force to overcome their mutual repulsion and force the nucleons to stay bound together. The preceding explanation shows the reason why it is easier to bombard a nucleus with neutrons than with protons. Since the neutrons have no charge, as they approach a positively charged nucleus they will not feel any repulsion. They therefore can easily "break" the electrostatic repulsion barrier to being exchanging mesons with the nucleus, thus becoming incorporated into it.


3 comments:
在你一無所有的時候 是誰在陪伴你 他便是你最重要的人 ............................................................
By: 品華 on 7 Juni 2010 pukul 21.38
變天了~~注意身體,別感冒囉! .................................................................
By: Anonim on 18 Juni 2010 pukul 15.31
時間就是塑造生命的材料。......................................................................
By: Anonim on 22 Agustus 2010 pukul 19.10
Posting Komentar